|
 Hungry. More Hungry. Can’t make it to lunch. Missed breakfast. Gotta go get coffee. But that means I’ll be by THAT food. That food they sell in 7-Elevens and little convenience stores like the one in my office. That food that isn’t ORGANIC. That food stuffed with nothing but High Fructose Corn Syrup and other things that you don’t need. Hungry. More Hungry. Can’t make it to lunch. Missed breakfast. Gotta go get coffee. But that means I’ll be by THAT food. That food they sell in 7-Elevens and little convenience stores like the one in my office. That food that isn’t ORGANIC. That food stuffed with nothing but High Fructose Corn Syrup and other things that you don’t need.
But I get coffee there everyday made by someone else who could be putting who knows what in it and it isn’t organic and I have to use that non-dairy creamer because they don’t stock soy and the creamer full of partially hydrogenated crap. Ah hell, hungry. Let’s eat. I go to the store and it’s a clear battle between a croissant freshly delivered and a muffin. I fought hard to avoid the section with the apple pastries, bear claw and Vienna filled Danishes. I thought, what goes good with coffee? It’s gotta be the muffins. And the chocolate chip one was chosen. Back at my computer I had a few gulps of coffee and starting chewing up the muffin. It’s been a while since I had food like this and it didn’t taste good. After a cleanse of crap that idea that food that is good for you tastes like crap, et el, was soon reversed. Since I wasn’t going to get any work done before I finished I decided to read the list of ingredients. It was like another language.
So with a little help of Webcrawler (no more Google since they decided to help a certain government block access to searches about human rights) here is what I found. And, it isn’t tasty. List of ingredients (I kid you not): Wheat & Malted Barley Flour (enriched with Niacin, Iron, Ascorbic Acid, Thiamine Mononitrate, Riboflavin & Folic Acid), Sugar, eggs, Canola Oil, Chocolate Chips (Sugar, Chocolate, Cocoa Butter, Butter Fat, Soy Lecithin, & Vanilla), Modified Food Starch, Soybean Oil, Whey, Leavening (Sodium Aluminum Phosphate, Aluminum Sulfate & Sodium Bicarbonate), Wheat Gluten, Salt, Propylene Glycol Monoesters, Mono & Diglycerides, Potassium Sorbate & Sodium Proponate, Natural & Artificial Flavors, Sodium Stearoyl Lactylate. I decided to go after the words in italics, especially the “bates.” Thiamine Mononitrate – Sounds weird, here’s what I found out: hiamine or thiamin, also known as vitamin1, is a colorless compound with chemical formula C12H17N4OS. It is soluble in water and insoluble in alcohol. Thiamine decomposes if heated. Thiamine was first discovered by Umetaro Suzuki in Japanrice bran cured patients of Beriberi. He named it aberic acid. Okay, not bad. B when researching how  Modified Food Starch – Anything “modified” sounded like I should check it out. Here’s what it is: Modified food starch is a starch that has been treated physically or chemically to modify one or more of its physical or chemical properties. The 'starch' could be from corn, wheat, potato, rice or tapioca--it depends on the manufacturer. By definition, modified food starch must contain less than .5% protein, but, it's up to the manufacturer to abide by that regulation, and there could be an exception. Modified Food Starch – Anything “modified” sounded like I should check it out. Here’s what it is: Modified food starch is a starch that has been treated physically or chemically to modify one or more of its physical or chemical properties. The 'starch' could be from corn, wheat, potato, rice or tapioca--it depends on the manufacturer. By definition, modified food starch must contain less than .5% protein, but, it's up to the manufacturer to abide by that regulation, and there could be an exception.
You are smart to read food labels. That is the best way to tell which ingredients are found in processed foods. However, to be even safer, always check with the manufacturer about specific ingredients that could pose a health risk. Now we’re getting somewhere.  Sodium Aluminum Phosphate – Aluminum is something people make baseball bats out of. Didn’t sound like something I want in my body. Here’s why: USES Sodium Aluminum Phosphate – Aluminum is something people make baseball bats out of. Didn’t sound like something I want in my body. Here’s why: USES
IN FOOD INDUSTRY:
The main use for SODIUM ALUMINUM PHOSPHATE, is as a leavening agent or acid for mixing baking powders, this is a new product in the baking industry. The SODIUM ALUMINUM PHOSPHATE, has a different performance profile than other leavening agents; it reacts slowly with the Sodium Bicarbonate in the mixing stage, there is only a 20 to 30 % Carbon Dioxide delivery from available. The difference is released during the oven stage. The SODIUM ALUMINUM PHOSPHATE also has an excellent buffering action for flour mixes, enhancing the properties of the formula ingredients. When using SODIUM ALUMINUM PHOSPHATE, the product is crunchy and has fine texture. Its use is very generalized. Aluminum Sulfate – Another: aluminum sulfate is a widely used industrial chemical. It is sometimes incorrectly referred to as alum, as it is closely related to this group of compounds. It occurs naturally as the mineral alunogenite. It is frequently used in the purification of drinking water supplies, and also in paper manufacturing.
Aluminum sulfate is rarely, if ever, encountered as the anhydrous salt. It forms a number of different hydrates, of which the hexadecahydrate is the most common.
It can also be very effective as a molluscicide, at killing Spanish slugs. Aluminum is used in water purification and as a mordant in dyeing and printing textiles. In water purification, it adsorbs impurities which are removed as the particulate settles to the bottom of the container.
When dissolved in a large amount of neutral or slightly-alkaline water, aluminum sulfate produces a gelatinous precipitate of aluminum hydroxide, Al(OH)3. In dyeing and printing cloth, the gelatinous precipitate helps the dye adhere to the clothing fibers by rendering the pigment insoluble.
Aluminum sulfate is the active ingredient of some antiperspirants; however, beginning in 2005 the US Food and Drug Administration no longer recognized it as a wetness reducer.
It is also used in styptic pencils. Sodium Bicarbonate – Anything with carbon (don’t they figure out the age of fossils with that?) had to be looked up: Sodium Bicarbonate, BICAR® , is a pure and synthetic product and is manufactured with the Solvay process which requires common salt and limestone. It is widely used in chemical and pharmaceutical industries, in animal feeds, in human food and other industrial and manufactured products. Solvay BICAR® is classified on different grades according its quality and the particle size. Particular grades are conform to European Pharmacopoeia and Food Chemicals Codex specifications. Pure & Synthetic? Come on. Still: SODIUM BICARBONATE [sodium bicarbonate] or sodium hydrogen carbonate, chemical compound, NaHCO 3 , a white crystalline or granular powder, commonly known as bicarbonate of soda or baking soda. It is soluble in water and very slightly soluble in alcohol. It evolves carbon dioxide gas when heated above about 50°C, a property made use of in baking powder, of which it is a component. It is also decomposed by most acids; the acid is neutralized and carbon dioxide is given off. The major use of sodium bicarbonate is in foods, e.g., baked goods. It is used in effervescent "salts" and is sometimes used medically to correct excess stomach acidity. It is also used in several kinds of fire extinguishers. Although it is an intermediate product in the Solvay process for making sodium carbonate , it is more economical to prepare it from purified sodium carbonate than to purify the intermediate. Because the bicarbonate is less soluble than the carbonate, carbon dioxide gas is bubbled into a saturated solution of pure carbonate, and the bicarbonate precipitates out to be collected and dried. Gluten – That word seems to always be coming up, so: Gluten is a form of protein found in many grains, such as wheat, barley, and rye. Propylene Glycol Monoesters – Propylene and Propane. Any connection? In assessing their cumulative effects, the fatty acid monoesters of glycerol and propylene glycol are members of a much larger class of compounds that are toxicologically and metabolically equivalent. All vertebrate systems deal with this class of compounds as food rather than toxicants. Glycerol fatty acid monoesters are natural components in dietary fats and natural breakdown products from metabolism of fat (triacylglycerol) in all living systems. Fatty acid esters of propylene glycol also occur as direct food additives in the human diet in substantial quantities. The use of fatty acid monoesters of glycerol and propylene glycol as pesticides will contribute a negligible amount (total U.S. population worst case estimate less than 0.2 mg/kg/day) to the existing cumulative exposure to the class of compounds when compared to natural levels of such compounds and their metabolites in tissue and foods (50-100 g/day in humans for glycerol esters), and to the levels permitted in food as direct additives (grams per day). Accordingly, exposure to these monoesters as a result of their label directed use as pesticides on raw agricultural food or feed commodities will result in a negligible increase in the cumulative exposure to this class of compounds over the present exposure, occurring as a result of daily consumption by the human population of this class of compounds from both naturally occurring sources and processed foods. VI. Determination of Safety for U.S. Population, Infants and Children 1. U.S. population. It is doubtful harm will result from aggregate exposure to residues of the fatty acid monoesters of glycerol or propylene glycol in the U.S. population. This includes all anticipated dietary exposures and all other exposures for which there is reliable information. The Agency has arrived at this conclusion based on the very low levels of mammalian toxicity (no toxicity at the maximum doses tested, Toxicity Category IV) associated with the fatty acid monoesters of glycerol and propylene glycol and the long history of their consumption. 2. Infants and children. FFDCA section 408 provides that EPA shall apply an additional tenfold margin of exposure (safety) for infants and children in the case of threshold effects to account for prenatal and postnatal toxicity and the completeness of the data base unless EPA determines a different margin of exposure will be safe for infants and children. Margins of exposure (safety) are often referred to as uncertainty factors. The registrant used the NOAEL of 1,000 mg/kg bwt/day determined in the 90-day oral toxicity study in rats to calculate an estimated exposure of the active ingredients to the U.S. population of 0.13 mg/kg bwt/day and to non-nursing infants of 0.44 mg/kg bwt/day. The corresponding margins of exposure were calculated to be 7,690 for the U.S. population and 2,270 for non-nursing infants (Ref. 2). In this instance, based on all the available information, the Agency concludes that the C8, C10, and C12 monoesters of glycerol and propylene glycol are virtually non-toxic to mammals, including infants and children. Further, the provisions of consumption patterns, special susceptibility, and cumulative effects do not apply. Since no toxic endpoints have been identified, any hazard is impossible to determine. As a result, EPA has not used a margin of exposure approach to assess the safety of the C8, C10, and C12 monoesters of glycerol and propylene glycol. Based on their abundance in nature and long history of use by humans without deleterious effects, there is reasonable certainty that no harm will result from aggregate exposure to the U.S. population, including infants and children, to residues of these glycerol and propylene glycol straight-chain fatty acid monoesters. This includes all anticipated dietary exposures and all other exposures for which there are reliable information. Thus, the Agency has determined that the additional margin of safety is not necessary to protect infants and children and that not adding any additional margin of safety will be safe for infants and children. Mono & Diglycerides – nitro glyserine anyone? Mono-diglycerides remain the most widely used emulsifiers in food production. They are called mono-diglrcerides because they are made from oils that have a high mono saturated fat content, but they are still hydrogenated. These are used in a wide variety of food manufacturing such as, breads, bagels, muffins, cookies, cakes, pies, donuts, pasta mixes, potato chips, ice creams, almost all packaged desserts, nearly all margarines and other spreads. Your local grocery bakery including Walmart bakeries use these oils. New margarine spreads Benecol™ and Take Control™ contain hydrogenated oils . Just read your label. Remember...its' your health! There are many food oil companies that produce mono-diglyerides. So when you read mono-diglycerides on packaged foods, it is nothing more than hydrogented oils. Below is a sample of one product listings from Gillco, oils manufactured by Quest International, one of the largest food oil producing companies worldwide. Gillco's url address is http://www.gillco.com. They sell mono-diglycerides using these names, Myverol®, Myvacet®, Myvatex®, Myvaplex®. 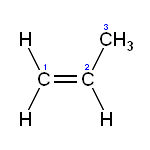 Potassium Sorbate – Sorbic Acid and Potassium Sorbate are preservatives that attack certain bacteria, molds and yeast. Potassium Sorbate – Sorbic Acid and Potassium Sorbate are preservatives that attack certain bacteria, molds and yeast.
Minimum concentrations of preservatives are determined by the pH and the water content of the foodstuffs to be preserved. The lower the pH value of the product the higher the acid and thus a lower amount of Sorbic Acid or Potassium Sorbate is needed for preservation.
Of course, Sorbic Acid can only protect product from attack by mold, yeast and bacteria if the food product has been processed under hygienic conditions. Already infected or partially perished food products cannot be protected through the addition of Sorbic Acid or Potassium Sorbate. Sorbic Acid and Potassium Sorbate are mold inhibitors. They inhibit mold from growing. They do not kill already established mold.
If food products are to be boiled intensively for long periods of time, it is recommended to add Sorbic Acid or Potassium Sorbate after the process to avoid its partial escape through evaporation of the water.
A basic idea to keep in mind when contemplating uses for Sorbic Acid or Potassium Sorbate is that Potassium Sorbate is water soluble and Sorbic Acid is soluble in fats, oils and some solvents but virtually insoluble in water.
Sorbic Acid is the active ingredient in both Sorbic Acid and Potassium Sorbate and although different rates could be used in a finished product, the results would be the same.
Sorbic Acid and Potassium Sorbate are considered by the Food and Drug Administration to be 11generally recognized as safe" (GRAS); therefore, the only limitations on their use is that the quantity not exceed the amount reasonably required to accomplish the desired preservative function. Food standards must also be consulted as they list the certain foods official specifications for certain standardized foods. In this case, only those ingredients listed in the standard and their respective amounts can be used.
GRAS chemicals can be used at any safe, suitable and reasonable levels in unstandardized foods to accomplish their functions.
State laws should be consulted which may differ from Federal regulations.
In all cases, when Sorbic Acid or Potassium Sorbate is used in a product, it must so state on the label.
Sodium Proponate – Sodium propionate is a transparent crystalline soluble substance used as a medical fungicide and to prevent the growth of moulds, especially to retard spoilage in packaged foods. & Sodium Stearoyl Lactylate – Sodium stearoyl lactate (and the similar calcium stearoyl lactate) is made by combining lactic acid and stearic acid, and then reacting the result with sodium hydroxide or calcium hydroxide to make the sodium or calcium salt. Replacing the lactic acid with fumaric acid gives sodium steroyl fumarate, a compound with same uses as the other two. Uses Sodium stearoyl lactylate is an emulsifier used as a dough strengthener in baked goods. It has several features that combine to make it very popular with bakers. It maintains the texture of fresh baked bread by keeping the amylose starch in its gelled state, preventing its recrystallization. It makes the gluten in the bread stronger and more extensible, increasing the volume of the loaf. It disperses the fats in the bread, making it softer, while allowing less fat to be used. It absorbs water, allowing the baker to get 1 to 1.5% more loaves from the same ingredients, thus making each loaf less expensive. It has a sweet taste, allowing less sugar to be used in the bread. Well, the muffin hit the spot and the coffee went down well. But I gotta tell you, I don’t think I will be eating any more of these anytime soon. Would you? PS – It was made by Tina’s, Inc. And called a “monster” muffin. Scary. Bring the Periodic Table of Elements next time.
Trackback(0)
|